Before being distributed on the global market, cosmetic products and medical devices, according to the regulations of their sector, shall be tested for evaluate their safety for human health. In particular, the European Regulation n.1223/2009 bans the animal experimentation of both finished cosmetic products and ingredients (or combinations of ingredients) intended to be contained in cosmetic products. Moreover, the European Union has been trying to turn it into a worldwide prohibition before 2023.
The contribution of specialized laboratories, such as UB-CARE, to evaluate the safe and efficacy of these kind of products through alternative methods, using cell cultures or 3D models, is becoming increasingly important.
Cytotoxicity test (MTT TEST)
 The MTT test is based on the ability of the MTT compound (tetrazolium salt, [3- (4,5-dimethylthiazol-2yl) -2,5-diphenyltetrazolium bromide) to be metabolized by the mitochondrial enzyme succinate dehydrogenase, thus constituting an indicator of cellular respiration. The reduction of MTT leads to the formation of crystals of a blue product insoluble in water, the formazan, whose quantity is proportional to the number of viable cells. The analysis can be performed according to ISO 10993-5 for medical devices “Biological evaluation of medical devices – Part 5: Tests for in vitro cytotoxicity”.
The MTT test is based on the ability of the MTT compound (tetrazolium salt, [3- (4,5-dimethylthiazol-2yl) -2,5-diphenyltetrazolium bromide) to be metabolized by the mitochondrial enzyme succinate dehydrogenase, thus constituting an indicator of cellular respiration. The reduction of MTT leads to the formation of crystals of a blue product insoluble in water, the formazan, whose quantity is proportional to the number of viable cells. The analysis can be performed according to ISO 10993-5 for medical devices “Biological evaluation of medical devices – Part 5: Tests for in vitro cytotoxicity”.
Market: Cosmetics, Medical devices, Raw materials, Household products.
Supported claim: Biosafety, Cytotoxicity.
Cytotoxicity test (NRU test)

The Neutral Red Uptake (NRU) test is based on the ability of neutral red dye (Neutral Red) to accumulate in cytoplasmic lysosomes by active transport that requires the integrity of cell membranes, providing information on their integrity and, indirectly, on cell viability. Through this analysis, a fine evaluation of the cytotoxic potential of the tested substances is obtained.
The analysis can be performed according to ISO 10993-5 for medical devices “Biological evaluation of medical devices – Part 5: Tests for in vitro cytotoxicity”.
Market: Cosmetics, Medical devices, Raw materials, Household products.
Supported claim: Biosafety, Cytotoxicity.
Cytotoxicity test (Kenacid Blue test)

This test evaluates the cytotoxicity of a product/raw material for cosmetic or medical use by the incorporation of a specific probe (Brilliant Blue) and the analysis of the total number of cells (biomass). The analysis can be performed according to the official protocol reported in the database DB-ALM (Database Service on Alternative Methods to animal experimentation), managed directly by the European Commission.
Market: Cosmetics, Medical devices, Raw materials, Household products.
Supported claim: Biosafety, Cytotoxicity.
Cell morphology
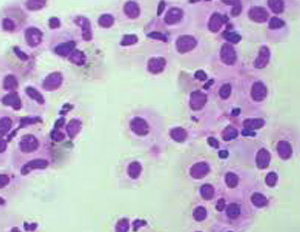
The effect on the cell morphology exerted by treatment with a finished product or raw material is evaluated using two dyes: the haematoxylin, which stains negatively charged cellular components (nucleic acids, membrane proteins) and the eosin which colours positively charged components (cytoplasm).
Market: Cosmetics, Medical devices, Raw materials.
Supported claim: Biosafety, Biocompatibility.
Cell structure
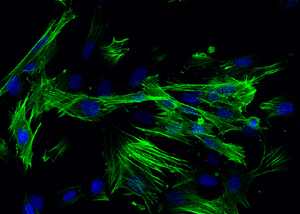
The test allows to analyse any cellular anomalies after treatment with raw materials or finished products. The analysis includes immunofluorescence labelling of proteins involved in the formation of cell cytoskeleton through specific antibodies linked to fluorescent molecules (fluorochromes).
Market: Cosmetics, Medical devices, Raw materials.
Supported claim: Biosafety, Biocompatibility.
Cell viability (LIVE-DEAD assay)
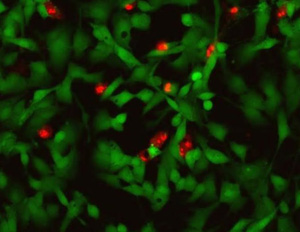
The test allows to evaluate the effect of treatment with a raw material or finished product for cosmetic or medical use on cell viability using due different probes: Syto 9 that stains the viable cells in green and the propidium iodide that highlights dead cells in red. The result is analysed in fluorescence microscopy.
Market: Cosmetics, Medical devices, Raw materials,
Supported claim: Biosafety, Cytotoxicity.
Skin sensitization (h-CLAT method)
 This test is able to evaluate the expression of two skin sensitization markers (CD86 and CD54) exposed on the cell surface of human immune cells (monocytes) following treatment with chemicals. The method, validated by the “European Union Reference Laboratory for Alternatives to Animal Testing (EURL ECVAM)” is performed following the OECD guidelines.
This test is able to evaluate the expression of two skin sensitization markers (CD86 and CD54) exposed on the cell surface of human immune cells (monocytes) following treatment with chemicals. The method, validated by the “European Union Reference Laboratory for Alternatives to Animal Testing (EURL ECVAM)” is performed following the OECD guidelines.
Market: Cosmetics, Medical devices, Raw materials, Household products.
Supported claim: Biosafety, Skin sensitization.
Cutaneous Corrosion 3D
 The test follows the procedure described in the OECD (Organization for the Economic Cooperation and Development) n. 431 (for the study of skin corrosion following contact with chemical raw materials). The study is carried out on 3D human epithelium reconstructed in vitro, which mimic the epidermis in each of its layers, where the product (solid or liquid) or extract is put in contact and then the cell viability is tested after different time of treatment.
The test follows the procedure described in the OECD (Organization for the Economic Cooperation and Development) n. 431 (for the study of skin corrosion following contact with chemical raw materials). The study is carried out on 3D human epithelium reconstructed in vitro, which mimic the epidermis in each of its layers, where the product (solid or liquid) or extract is put in contact and then the cell viability is tested after different time of treatment.
Moreover, the cytokine IL-1α, as irritation marker, can be evaluated, investigating its levels in the supernatant of 3D epidermis inserts.
Market: Cosmetics, Medical devices, Raw materials, Household products.
Supported claim: Biosafety, Cytotoxicity, Irritation.
Phototoxicity

Phototoxicity means the toxic response that occurs after primary exposure of the skin to some chemicals in the presence and in the absence of exposure to a non-cytotoxic dose of simulated solar light.
Through this test it is possible to identify the compounds that could be phototoxic in vivo after systemic application and distribution on the skin and the compounds with photoirritating effect after local application on the skin. The method is validated (OECD 432 guideline).
Market: Cosmetics, Medical devices, Raw materials, Household products.
Supported claim: Biosafety, Cytotoxicity, Phototoxicity.
Cutaneous irritation 3D test
The test follows the procedure described in the ISO 10993-10 standard and validated by ECVAM for the study of skin irritation following contact with chemical raw materials (OECD n. 439). The study is carried out on 3D human epithelium reconstructed in vitro, which mimic the epidermis in each of its layers, where the product or extract is put in contact and then the cell viability is tested.
Moreover, the cytokine IL-1α, as irritation marker, can be evaluated, investigating its levels in the supernatant of 3D epidermis inserts.
Market: Cosmetics, Medical devices, Raw materials, Household products.
Supported claim: Biosafety, Cytotoxicity, Irritation.
Mutagenicity test (Ames test)
 The identification of substances capable of inducing mutations is crucial for the assessment of their safety, because they could damage germ cell line DNA. Gene mutations are easily identifiable in bacteria because they can cause changes in growth dependent on the nutrients needed for development. The Ames test is widely used to identify compounds. The Ames test is widely used to identify compounds that are capable of inducing gene mutations, using five different Salmonella strains (ISO 10993-3 e la OECD n.471).
The identification of substances capable of inducing mutations is crucial for the assessment of their safety, because they could damage germ cell line DNA. Gene mutations are easily identifiable in bacteria because they can cause changes in growth dependent on the nutrients needed for development. The Ames test is widely used to identify compounds. The Ames test is widely used to identify compounds that are capable of inducing gene mutations, using five different Salmonella strains (ISO 10993-3 e la OECD n.471).
Market: Cosmetics, Medical devices, Raw materials.
Supported claim: Biosafety, Non-mutagenicity.
Carcinogenicity test
 This test evaluates the potential carcinogenic effect of a substance tested in murine cells (fibroblasts BALB/c 3T3) to induce morphological changes typical of the transition from normal to cancerous form. In fact, the phenomenon of morphological transformation of cells leads to changes in the behaviour and control of growth in culture, as well as to a disorganized growth pattern and to the acquisition of anchorage-dependent growth capacity. This test is performed follow the protocol designed in ECVAM (European Commission Validation Alternative Methods).
This test evaluates the potential carcinogenic effect of a substance tested in murine cells (fibroblasts BALB/c 3T3) to induce morphological changes typical of the transition from normal to cancerous form. In fact, the phenomenon of morphological transformation of cells leads to changes in the behaviour and control of growth in culture, as well as to a disorganized growth pattern and to the acquisition of anchorage-dependent growth capacity. This test is performed follow the protocol designed in ECVAM (European Commission Validation Alternative Methods).
Market: Medical devices, Raw materials.
Supported claim: Biosafety, Carcinogenicity, Mutagenicity.
Gastric irritation
In order to evaluate the possible irritant effect of a raw material, medical device or food supplement, the cell viability is assessed on cell cultures deriving from gastric mucosae, in order to recreate in vitro the ideal conditions for a result comparable to what happens in the gastric mucosa.
Market: Medical devices, Raw materials, Food supplement
Supported claim: Cytotoxicity, Irritation.
Ocular irritation 3D test
 The test evaluates the potential irritant action of a chemical compound against the ocular epithelium, through the use of a reconstructed three-dimensional human corneal epithelium model (HCE, SkinEthicTM). The assay quantitatively analyses cell viability by MTT assay following the application of a raw material or a finished product for cosmetic or medical use on the in vitro model surface
The test evaluates the potential irritant action of a chemical compound against the ocular epithelium, through the use of a reconstructed three-dimensional human corneal epithelium model (HCE, SkinEthicTM). The assay quantitatively analyses cell viability by MTT assay following the application of a raw material or a finished product for cosmetic or medical use on the in vitro model surface
Market: Cosmetics, Medical devices, Raw materials, Household products
Supported claim: Ocular irritation.
Vaginal irritation 3D test
This test is focused on the study of vaginal irritation following contact with a raw material or finished products. The analysis is performed on a 3D vaginal epithelium (HVE, SkinEthicTM) reconstructed in vitro, which mimic the epithelium in each of its layers. The cell viability of the insert is tested after direct contact with the product to be tested.
Market: Cosmetics, Medical devices, Raw materials.
Supported claim: Vaginal irritation
Genotoxicity test (Micronucleus)
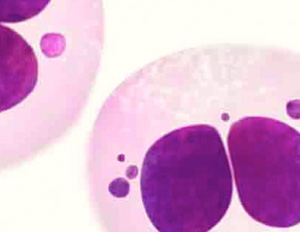 The micronucleus assay (OECD 487) is normally used as a genotoxicity screening; it is used to identify those substances capable to induce a damage to chromosomes that leads to the formation of micronuclei in the interphase cells. Micronuclei are bodies containing chromatin that may represent part or whole chromosomes that do not migrate correctly during the cell cycle anaphase.They can form as a result of the action of clastogenic agents, which induce cracks in the chromosomes mainly through interaction with the DNA, or aneugenes, which induce the loss/acquisition of chromosomes by interaction with the mitotic spindle.
The micronucleus assay (OECD 487) is normally used as a genotoxicity screening; it is used to identify those substances capable to induce a damage to chromosomes that leads to the formation of micronuclei in the interphase cells. Micronuclei are bodies containing chromatin that may represent part or whole chromosomes that do not migrate correctly during the cell cycle anaphase.They can form as a result of the action of clastogenic agents, which induce cracks in the chromosomes mainly through interaction with the DNA, or aneugenes, which induce the loss/acquisition of chromosomes by interaction with the mitotic spindle.
Market: Medical devices, Raw materials.
Supported claim: Genotoxicity.
